The Nitrogen cycle—another natural cycle we've altered
Nitrogen is an essential element for all life, as it's a major component of amino acids (proteins) and nucleic acids (DNA and RNA). The main pool of nitrogen is the atmosphere, comprising 78% of the atmosphere as nitrogen gas (N2). Unfortunately, plants cannot use N2 directly. It must be converted to either ammonium (NH3) or nitrate (NO3-) before plants can absorb it and incorporate it into amino acids and nucleic acids. In nature, most nitrogen conversion is done through a process known as nitrification wherein N2 is converted first to NH3 by several genera of nitrogen-fixing bacteria and cyanobacteria, then to NO2- (nitrite) by bacteria in the genera Nitrosomonas or Nitrococcus, and finally to NO3- by bacteria in the Nitrobacter genus. Nitrate that isn't absorbed by plants is returned to the atmosphere by the process of denitrification, wherein nitrate is converted to N2 gas by denitrifying bacteria.
After waste (like urea) is excreted or a plant or animal dies, nitrogen in their tissues is converted back to ammonia by bacteria and fungi via a process called ammonification (also known as mineralization). Most of that ammonia is recycled into nitrate, where it is again absorbed by plants or returned to the atmosphere by denitrification. Other sources of nitrogen-fixation include lightening strikes and ultraviolet radiation, both of which produce nitric acid (HNO3) in the atmosphere, which then settles out on the ground.
How are humans altering this cycle? Mostly by artificially fixing nitrogen gas into ammonium and nitrate by the Haber process. This process was first used to create nitrate for explosives during WWI and WWII. After WWII, it was used to create fertilizer for the Green Revolution. Second, burning fossil fuels, especially coal and gasoline, produces N2O and NOx, which form nitric acid and smog. Third, we've planted literally thousands of acres of legumes, a family of plants including soybeans and peanuts as well as trees like black locust and honey locust. Legumes have nodules containing nitrogen-fixing bacteria on their roots. Planting legumes increases the amount of fixed nitrogen in the soil, much like adding fertilizer. Finally, we ourselves and our farm animals produce large amounts of urea as waste. That isn't so bad except we tend to concentrate ourselves in cities and our animals in feedlots and factory farms, meaning that the amount of urea is concentrated as well.
Taken as a whole, humans have have dramatically increased the amount of fixed nitrogen, from ~15,000,000 metric tons per year in 1860 to 187,000,000 metric tons per year in 2005 (see Vitousek et al. 1997; Fields 2004; Galloway et al. 2008 (downloadable pdf available here)). By comparison, most estimates of natural nitrogen fixation come in around 125,000,000 metric tons. Nitrogen use is widespread around the planet, particularly in the US, Europe, and southeastern Asia—the main agricultural areas of the world.
The consequences are widespread. Chief among them are dead zones in lakes, ponds, and the ocean. Excess nitrates are washed off the land into waterways. Algae and blue-green algae (also called cyanobacteria) in streams, lakes, and rivers are normally nitrogen-limited, so an influx of nitrate and ammonia induces major growth in algae populations. Algae have one major limit: The population will grow exponentially until all the excess nitrogen is used up. Once that happens, the algae die and bacteria decompose their remains. Decomposition requires oxygen, which depletes the dissolved oxygen supply in the water. No oxygen = a dead zone, like the infamous dead zone in the Gulf of Mexico, the largest such zone in the world.
Cyanobacteria blooms don't deplete oxygen like a bloom of green algae eventually will. Instead, cyanobacteria produce a neurotoxin which at high concentrations can kill animals and sicken humans who try to swim in the water. Two recent examples, both from the US state of Ohio, include Grand Lake Saint Mary and the western end of Lake Erie, near Toledo, Ohio.
Other impacts are less noticeable. In areas where nitrogen has been severely limited (certain sand plains, sand hills, etc), increased nitrogen will alter the type of plants that can grow in those areas, driving out the vegetation native to that area. In other soil that is already nitrogen-rich, excess nitrogen will acidify the soil, altering the types of minerals such as calcium or magnesium available to plants. Excess nitrate can also percolate down to groundwater, with negative impacts on human health.
So, what can be done about it? After all, we're not going to stop using nitrogen fertilizers—our food supply depends on them. There are three main solutions. First, changing crop rotation patterns to include more perennial species can cut fertilizer use by some 90% (Davis et al. 2012; Blesh and Drinkwater 2013). Second, forest buffers along streams can cut the amount of nitrogen in run-off by 90% (i.e. Lowrance et al. 1984; Brian et al. 2004). However, to be truly effective, those buffers must be at least 30 meters (98 feet) wide. Third, reducing fossil fuel use and/or installing better scrubbers on coal plants and catalytic converters on automobiles would reduce nitrogen pollution even more than it already has been.
Further reading:
Schlesinger, W. H. 2008. On the fate of anthropogenic nitrogen. PNAS 106:203-208.
Rockström, J. et al. 2009. A safe operating space for humanity. Nature 461:472-475
After waste (like urea) is excreted or a plant or animal dies, nitrogen in their tissues is converted back to ammonia by bacteria and fungi via a process called ammonification (also known as mineralization). Most of that ammonia is recycled into nitrate, where it is again absorbed by plants or returned to the atmosphere by denitrification. Other sources of nitrogen-fixation include lightening strikes and ultraviolet radiation, both of which produce nitric acid (HNO3) in the atmosphere, which then settles out on the ground.
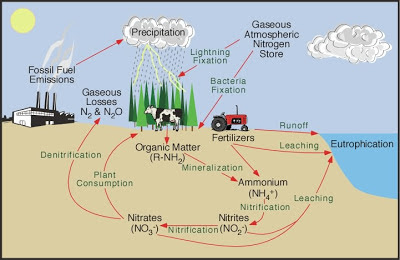 |
| Diagram from http://www.physicalgeography.net/fundamentals/9s.html |
How are humans altering this cycle? Mostly by artificially fixing nitrogen gas into ammonium and nitrate by the Haber process. This process was first used to create nitrate for explosives during WWI and WWII. After WWII, it was used to create fertilizer for the Green Revolution. Second, burning fossil fuels, especially coal and gasoline, produces N2O and NOx, which form nitric acid and smog. Third, we've planted literally thousands of acres of legumes, a family of plants including soybeans and peanuts as well as trees like black locust and honey locust. Legumes have nodules containing nitrogen-fixing bacteria on their roots. Planting legumes increases the amount of fixed nitrogen in the soil, much like adding fertilizer. Finally, we ourselves and our farm animals produce large amounts of urea as waste. That isn't so bad except we tend to concentrate ourselves in cities and our animals in feedlots and factory farms, meaning that the amount of urea is concentrated as well.
Taken as a whole, humans have have dramatically increased the amount of fixed nitrogen, from ~15,000,000 metric tons per year in 1860 to 187,000,000 metric tons per year in 2005 (see Vitousek et al. 1997; Fields 2004; Galloway et al. 2008 (downloadable pdf available here)). By comparison, most estimates of natural nitrogen fixation come in around 125,000,000 metric tons. Nitrogen use is widespread around the planet, particularly in the US, Europe, and southeastern Asia—the main agricultural areas of the world.
 |
| From Galloway et al. (2008). |
Cyanobacteria blooms don't deplete oxygen like a bloom of green algae eventually will. Instead, cyanobacteria produce a neurotoxin which at high concentrations can kill animals and sicken humans who try to swim in the water. Two recent examples, both from the US state of Ohio, include Grand Lake Saint Mary and the western end of Lake Erie, near Toledo, Ohio.
Other impacts are less noticeable. In areas where nitrogen has been severely limited (certain sand plains, sand hills, etc), increased nitrogen will alter the type of plants that can grow in those areas, driving out the vegetation native to that area. In other soil that is already nitrogen-rich, excess nitrogen will acidify the soil, altering the types of minerals such as calcium or magnesium available to plants. Excess nitrate can also percolate down to groundwater, with negative impacts on human health.
So, what can be done about it? After all, we're not going to stop using nitrogen fertilizers—our food supply depends on them. There are three main solutions. First, changing crop rotation patterns to include more perennial species can cut fertilizer use by some 90% (Davis et al. 2012; Blesh and Drinkwater 2013). Second, forest buffers along streams can cut the amount of nitrogen in run-off by 90% (i.e. Lowrance et al. 1984; Brian et al. 2004). However, to be truly effective, those buffers must be at least 30 meters (98 feet) wide. Third, reducing fossil fuel use and/or installing better scrubbers on coal plants and catalytic converters on automobiles would reduce nitrogen pollution even more than it already has been.
Further reading:
Schlesinger, W. H. 2008. On the fate of anthropogenic nitrogen. PNAS 106:203-208.
Rockström, J. et al. 2009. A safe operating space for humanity. Nature 461:472-475



Comments
Post a Comment